Medical device inspection, testing & failure analysis
From surgical tools to implantable devices, patients count on medical providers to use equipment that is safe, reliable, and free of contaminants.
Offering state-of-the-art laboratories and a nationwide reach, our Lab Services division is well equipped to ensure mechanical integrity and safe material properties of your medical instruments and implant materials. Our capabilities include: Reverse Engineering, Comparative CAD Analysis, Dimensional Metrology, Laser & CT Scanning, Chemical Testing, Mechanical Testing, Metallurgical Testing, Non-Destructive Testing, and more. Our labs also feature EMI, EMC and Calibration capabilities.
NEED A QUOTE?
Simply fill out your name, number, and email below and someone from our team will contact you within 24 hours.
Failure Analysis
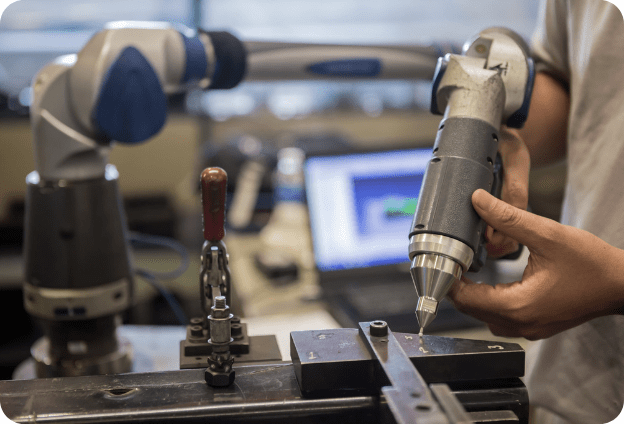

Our deep understanding of testing and inspection — and state-of-the-art equipment — ensures highly accurate results. With decades of collective experience, our experts know which techniques will best meet your unique challenge.
Beyond the analysis, our team is available to develop a comprehensive corrective action plan to ensure that your medical product is 100% safe before it re-enters the market.
Fluid Testing & Analysis
Our ISO-accredited labs provide a full menu of fluid testing and analysis to monitor the health of equipment and machinery used by medical device manufacturers. Our ASTM-compliant services include testing and analysis of lubricating oil, fuel, coolant, grease, transformer oils, and other equipment fluids.
Why IIA?
Partner with IIA Lab Services for:
- Faster turnaround times
- Accurate Data/ Reliable Results
- Cost Containment
- Responsive Customer Service

From product certification to failure analysis, we deliver the answers you need to move forward safely and with confidence.
CHEMICAL ANALYSIS SERVICES
- Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES)
- Optical Emission Spectroscopy (OES)
- X-Ray Fluorescence Spectroscopy (XRF, PMI)
- Pyrolysis Gas Chromatography Mass Spectroscopy (PyGC/MS)
- Fourier Transform Infrared Spectroscopy (FTIR)
- Thermographic Analysis (TGA)
- Differential Scanning Calorimetry (DSC)
- Nickel Ion Release Testing
- Particulate Testing
- Phthalates and Latex
- CPSIA/CPSC Heavy Metals
METALLURGICAL TESTING SERVICES
- Failure Analysis
- Scanning Electron Microscopy (SEM)
- Energy Dispersive Spectroscopy (EDS)
- Coating/Plating Thickness and Evaluations
- Microstructure Analysis
- Grain Size
- Microhardness
- Immersion Corrosion Evaluations
- Surface Roughness Measurement
WIRELESS & TELECOMMUNICATION EMC/EMI TESTING SERVICES
- EMF/RF Exposure & SAR testing
- Electrical/Product Safety Testing
- ISO/IEC 17065: 2012 Accredited Product Certification Body for U.S. FCC, ISED Canada, MIC Japan, IMDA Singapore. Notified/Approved Body (NB/AB1177) for the EU Radio Equipment and EMC Directives and the United Kingdom Radio Equipment and EMC Regulations.
MECHANICAL TESTING SERVICES
- Tension Testing (Tensile, Yield, Elongation, Reduction of Area, Modulus, Stain Hardening Exponent)
- Compression Testing
- Shear Testing
- Hardness (Rockwell, Brinell, Vickers, Durometer, Pencil, Leeb)
- Impact Testing (Charpy, Izod, Gardner)
- Tear Strength
- Flexural Properties
ENGINEERING SUPPORT SERVICES
- CT & Laser Scanning
- Part-to-CAD Comparison
- 3D Dimensional Inspection
- Geometry Recreation (Reverse Engineering)
DIMENSIONAL INSPECTION SERVICES
- First Article Inspection
- Capability Studies
- Supplier Validation (3rd Party Inspection)
- Mold & Fixture Evaluation
- CMM training on PCDMIS and Calypso
- FARO laser scanners training
Resources
Resources
Additional Resources



Certifications and Accreditations
Basic Safety and Essential Performance of Medical Electrical Equipment, Medical Electrical Systems, and Laboratory Medical Equipment. We are pleased to offer our expertise and can work closely with you on FCC or ISED communications regarding product certification. We have a deep understanding of the testing and inspection industry, and our experts are adept at knowing which modalities to use to provide a fast, viable, and high-quality solution to your challenge.
All inspectors are certified to one or more of the following:
- CQI (Certified Quality Inspector)
- CMI (Certified Mechanical Inspector)
- CQT (Certified Quality Technician)
- CQE (Certified Quality Engineer)
- CQM (Certified Quality Manager)
- CQA (Certified Quality Auditor)
- Duns Number:19-993-4829
- NAICS: 541380 (Mech. Testing Lab or Services)
- Cage Code: 0GB04
- SIC Code: 8734


